Desalination Chlorination
Description
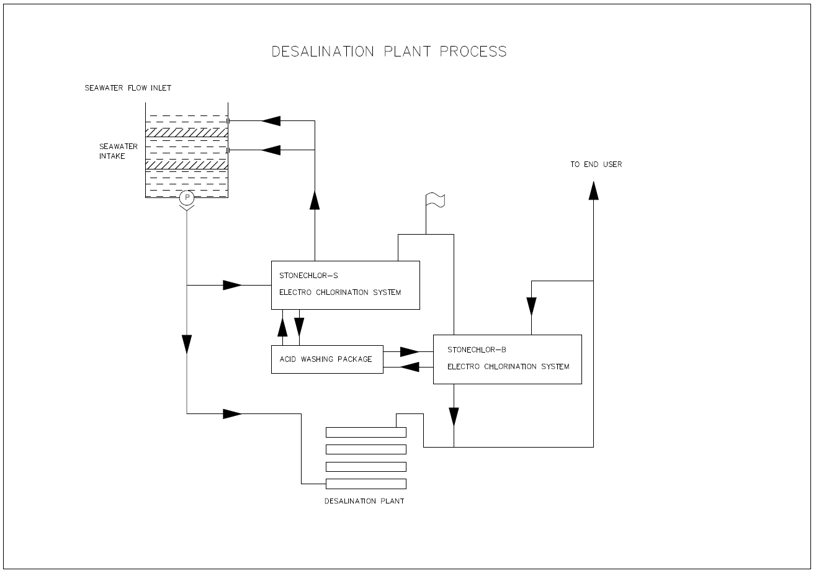
Desalination chlorination requires various chlorination technologies application.Desalination plant required seawater for its reverse osmosis process to generate fresh water, seawater or brine electro chlorination system is required to generate sodium hypochlorite to be injected at seawater intake structure for biological anti fouling purpose.
After seawater desalination process, disinfection by sodium hypochlorite is needed, brine sodium hypochlorite generation system could generate sodium hypochlorite from sodium chloride (NaCl), and the generated Sodium Hypochlorite will be injected into the fresh water line from the desalination plant.
Based on Milestone Chlorination Technologies LLC years engineering for water treatment experienced is able to provide desalination chlorination application total solution for clients such as intake structure chlorination injection design and downstream disinfection design. For land based application such as this desalination chlorination industry, we will use Parallel Plates Electrolyzers with acid washing packages for electro chlorination system to achieve capital and operation cost effective.
Process Flow
The fresh water goes into the package through the water softener to eliminate calcium and magnesium in it, the solid salt (NaCl) pulls into salt saturator mixed with fresh water becomes saturated brine, the saturated brine pump delivers the saturated brine and the fresh water pump transfer the fresh water mixed with saturated brine mixed into diluted brine (2.0% to 3.5%), the diluted brine goes into the electrolyzer assembly, which has DC power supplies by the transformer rectifier to it.The rectifier for small and normal capacity we designed is using switch mode, which is more cost effective and small foot print, also the rectifier is specifically for the requirements of the installed electrolytic cell. It converts the AC platform input voltage into the required low voltage DC current and regulates this output to a selected and controlled level.
Electrolysis process takes place within the electrolytic cell as per the following equation:
At the anode: 2 Cl - 2 e → Cl2
At the cathode: 2 Na+ 2 H2O + 2 e→ 2 NaOH + H2
Overall: 2 NaCl + 2 H2O → 2 NaOCl + 2 H2
Through the electrolysis process the package produces the required amount of sodium hypochlorite together with the by-product, hydrogen gas.
The solution with hydrogen gas are fed into degassing tank and the hydrogen gas will be pushed out by air blowers connected with the storage tank to vent to open atmosphere.
The hydrogen free solution containing sodium hypochlorite is then dosed into the dosing point through dosing pumps or gravity at certain pressure.




 English
English 


